
Tellurium-Ligated Pd(II) Complex of Bulky Organotellurium Ligand as a Catalyst of Suzuki coupling: First Report on In Situ Generation of Bimetallic Alloy 'Telluropalladinite' (Pd9Te4) Nanoparticles and Role in Highly Efficient Catalysis

Palladium complexes bearing pyridylthioether ligands. Synthesis and application as efficient phosphine-free catalysts in Suzuki-Miyaura couplings - ScienceDirect

Facile and efficient protocols for C–C and C–N bond formation reactions using a superparamagnetic palladium complex as reusable catalyst | SpringerLink
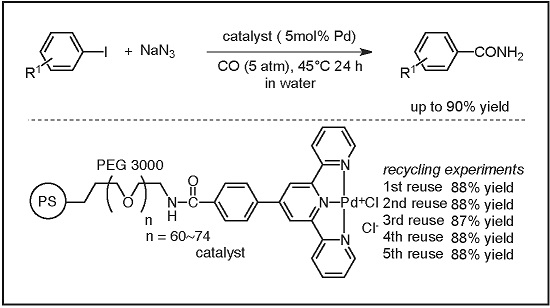
Catalysts | Free Full-Text | Recyclable Polymer-Supported Terpyridine–Palladium Complex for the Tandem Aminocarbonylation of Aryl Iodides to Primary Amides in Water Using NaN3 as Ammonia Equivalent
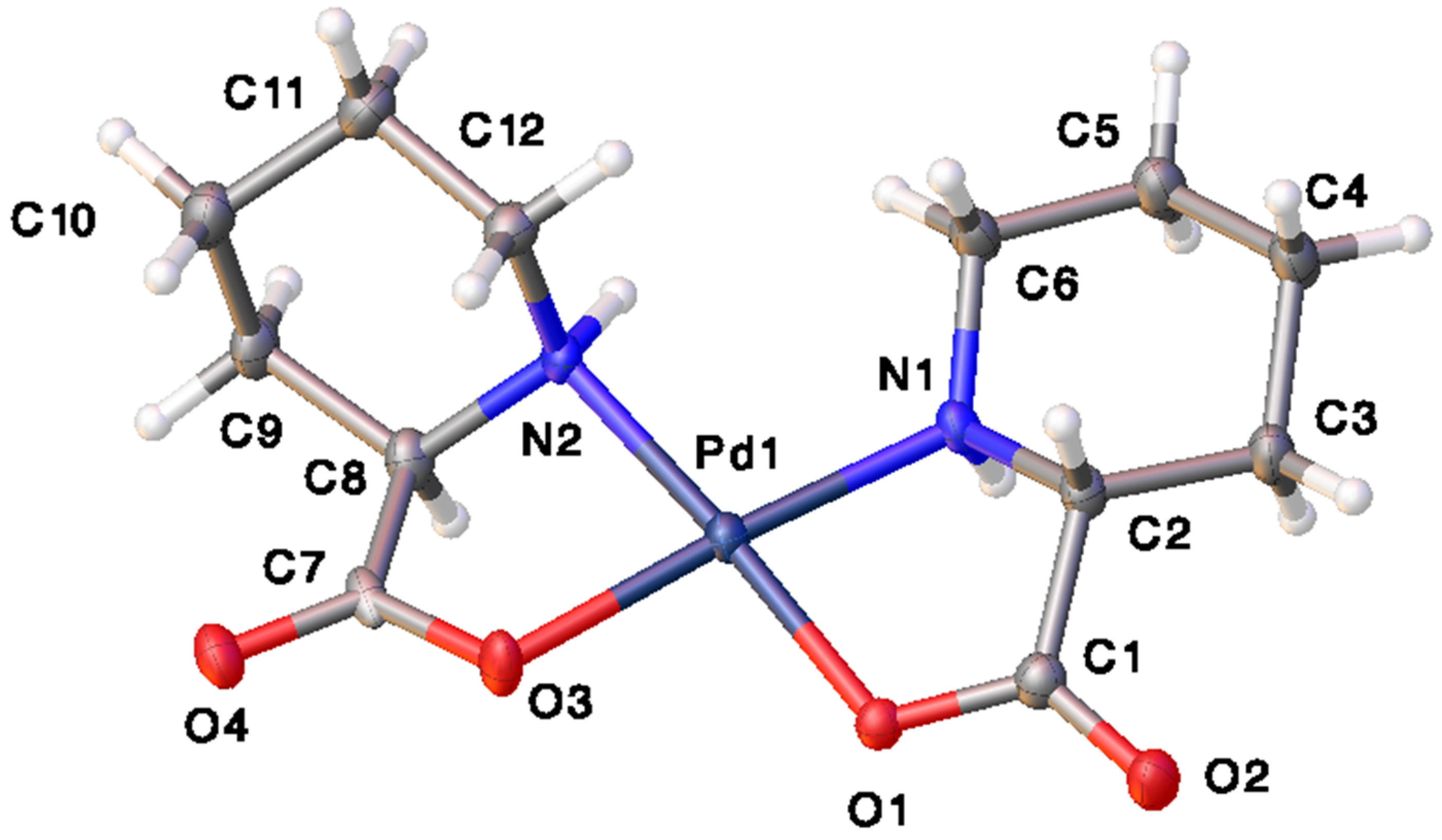
Catalysts | Free Full-Text | Synthesis, Structure, and Catalytic Reactivity of Pd(II) Complexes of Proline and Proline Homologs
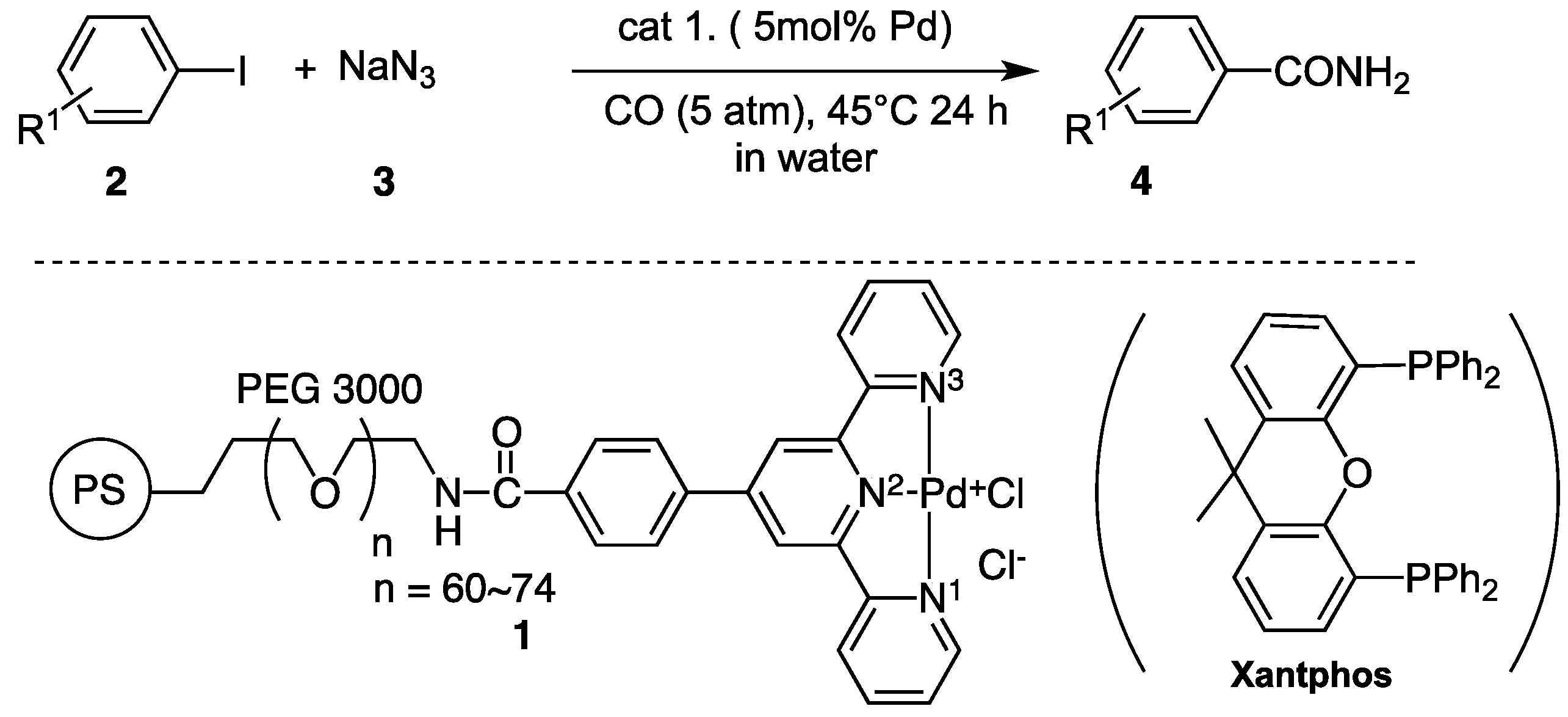
Catalysts | Free Full-Text | Recyclable Polymer-Supported Terpyridine–Palladium Complex for the Tandem Aminocarbonylation of Aryl Iodides to Primary Amides in Water Using NaN3 as Ammonia Equivalent
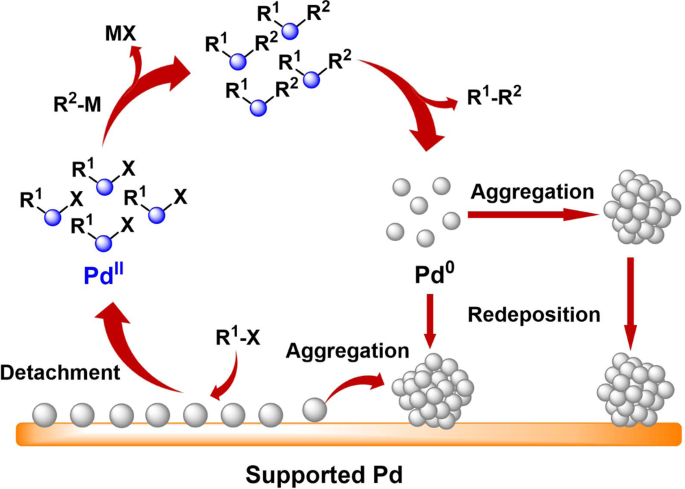
Enhancing stability by trapping palladium inside N-heterocyclic carbene-functionalized hypercrosslinked polymers for heterogeneous C-C bond formations | Nature Communications
![Palladium Catalysts [Cross-coupling Reaction using Transition Metal Catalysts] | Tokyo Chemical Industry (India) Pvt. Ltd. Palladium Catalysts [Cross-coupling Reaction using Transition Metal Catalysts] | Tokyo Chemical Industry (India) Pvt. Ltd.](https://www.tcichemicals.com//assets/cms-images/category12640-2.png)
Palladium Catalysts [Cross-coupling Reaction using Transition Metal Catalysts] | Tokyo Chemical Industry (India) Pvt. Ltd.
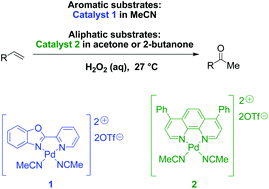
Cationic palladium(ii) complexes as catalysts for the oxidation of terminal olefins to methyl ketones using hydrogen peroxide - Green Chemistry (RSC Publishing)

Understanding the Unusual Reduction Mechanism of Pd(II) to Pd(I): Uncovering Hidden Species and Implications in Catalytic Cross-Coupling Reactions. | Semantic Scholar

Synthesis, Characterization, and Catalysis of Water‐Soluble Trimeric and Monomeric Palladium Complexes of 8‐Aminoquinolines - Dolui - 2023 - European Journal of Inorganic Chemistry - Wiley Online Library
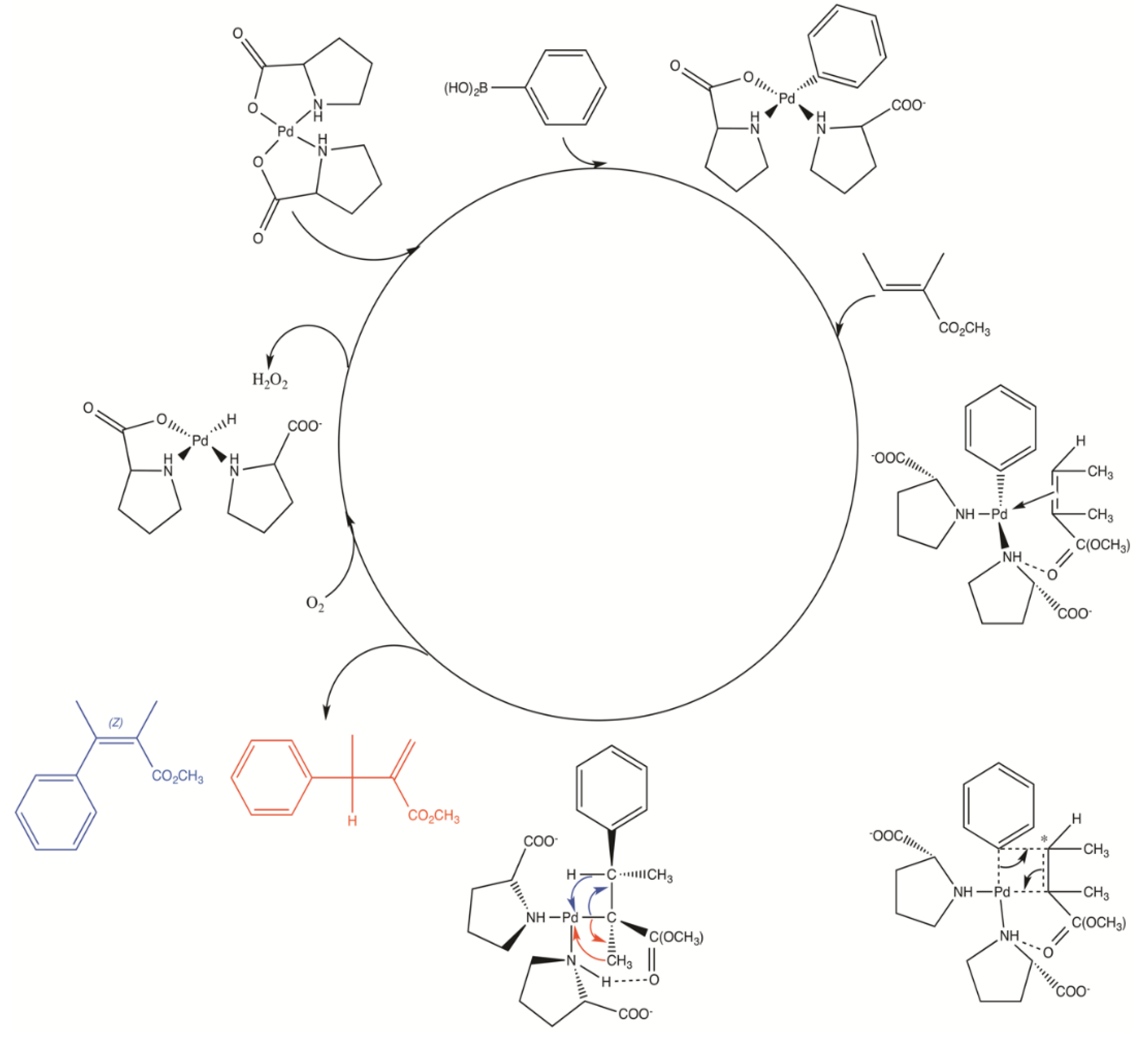
Catalysts | Free Full-Text | Synthesis, Structure, and Catalytic Reactivity of Pd(II) Complexes of Proline and Proline Homologs

Palladium(II) complexes featuring bidentate pyridine–triazole ligands: Synthesis, structures, and catalytic activities for Suzuki–Miyaura coupling reactions - ScienceDirect
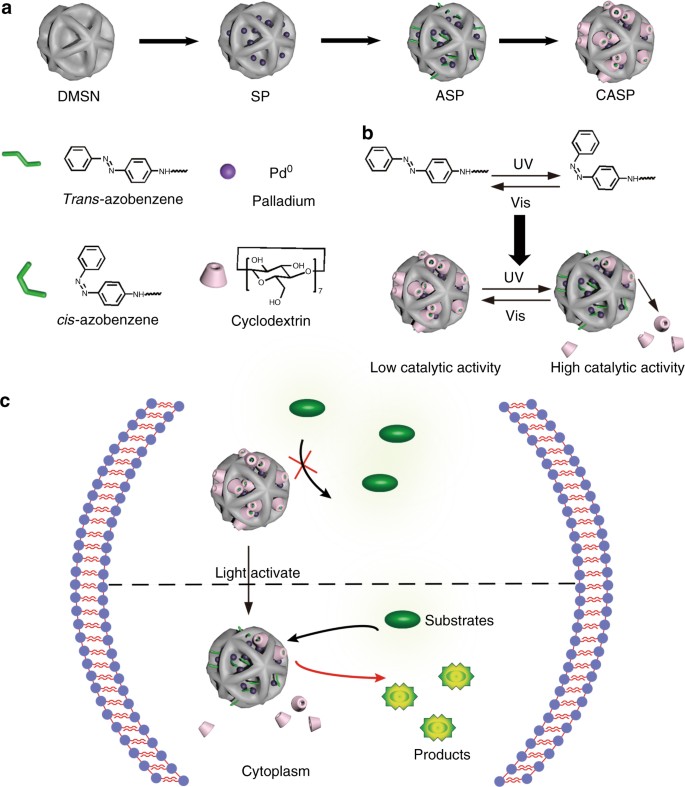
Designed heterogeneous palladium catalysts for reversible light-controlled bioorthogonal catalysis in living cells | Nature Communications
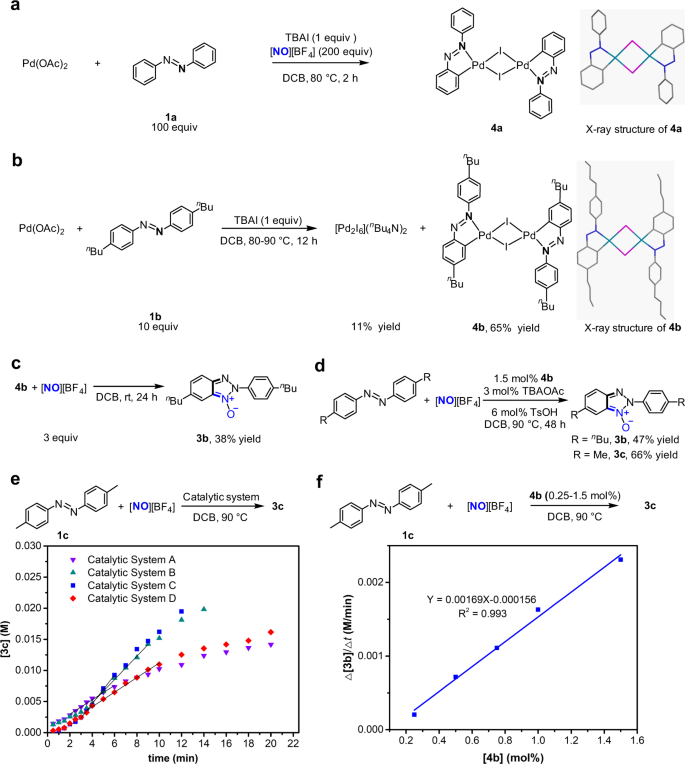
Iodide-enhanced palladium catalysis via formation of iodide-bridged binuclear palladium complex | Communications Chemistry

Benzimidazolyl Palladium Complexes as Highly Active and General Bifunctional Catalysts in Sustainable Cross-Coupling Reactions | ACS Catalysis

The first use of porphyrins as catalysts in cross-coupling reactions: a water-soluble palladium complex with a porphyrin ligand as an efficient catalyst precursor for the Suzuki–Miyaura reaction in aqueous media under aerobic
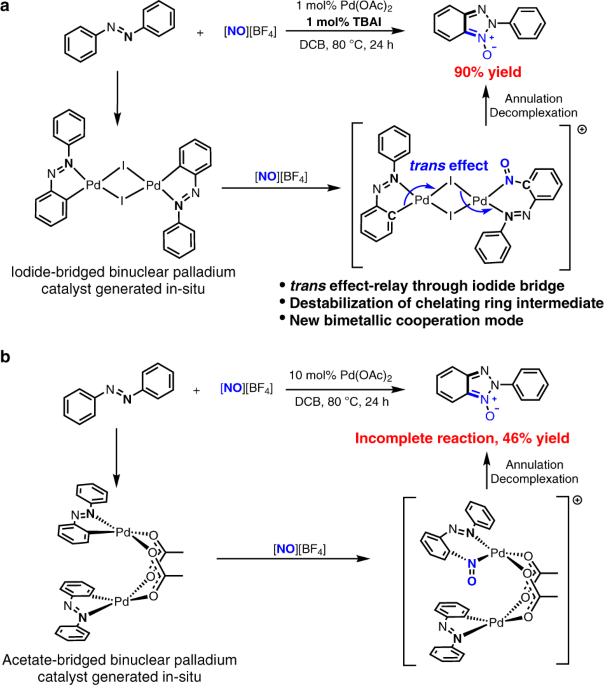
Iodide-enhanced palladium catalysis via formation of iodide-bridged binuclear palladium complex | Communications Chemistry

Interplay of Supramolecular Chemistry and Photochemistry with Palladium-Catalyzed Ethylene Polymerization | CCS Chemistry

Catalysts MDPI on X: "Synthesis of MCM-41 #Immobilized (Phenoxy)Imine # Palladium(II) #Complexes as #Recyclable Catalysts in the #Methoxycarbonylation of 1-Hexene 📝by Saphan O. Akiri and Stephen O. Ojwach. 👉https://t.co/XhKAnc9CW1 https://t.co ...








